Hi,
ok, i have posted about this topic a few times in the past, and am hoping someone with chemistry knowledge might be able to solve a mystery
why is it, when using Pickle-It, the sterling silver metal gets a dull grey stain on the surface? and then darker grey splotches after being submersed in a baking soda/ water neutralizing bath?
here is what i have done so far:
i annealed a sterling silver ingot
i used boric acid/ denatured alcohol as a barrier flux
i quenched in room tempurature water
i cleaned in hot water in a crock pot (set to high)
i rinsed it in tap water
the ingot came out clean
then i put the ingot in Pickle-It in a crock pot (set to low)
after a minute, it turned a uniform dull light grey
Pickle-It was a 1-8 mix ratio with water
then i took the ingot out of the Pickle-It, and put it directly imto a bath of 1 Tablespoon of baking soda and about 3 cups if water
the ingot immediately started to stain a splotchy darker grey
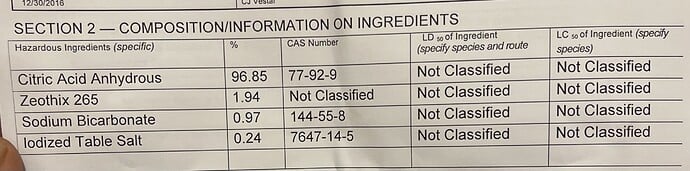
i took a look at the MSDS for it
i noticed under the section “reactivity” it says “yes” to “strong bases- oxidizing agents”
does this have something to do with the reaction i am seeing?
the ingredients listed are:
any thoughts?
i am sooo curious…
julie