Platinum has a higher melting point than iron or steel, so a furnace that can melt platinum is really not the sort of thing you’d want in a home shop.The heat needed to melt more than the tiniest amount of platinum is far beyond what any bench-top torch can produce.
You can weld platinum with a small torch because you’re only melting the metal in immediate contact with the seam, but melting enough to pour a small ingot requires a much bigger heat source. Propane, or natural gas, and oxygen gets hot enough, but the torch needs to put out a lot of heat all at once. Most shops will have large torches specifically for casting scrap into ingots.
I have spent 50 years just working in Sterling Silver and occasionally Gold Copper and Brass…Rob
I can’t recommend a specific torch… if you are melting a large quantity of silver, your torch and tips have to be able to handle a high gas flow and put out enough heat energy (BTUs or Joules per unit time) to heat the metal and keep it molten with some superheat. I’ve done virtually all of my fabrication work using a standard cheap hardware store propane/air torch. For melting and pouring I’ve used an oxyacetylene torch with a cutting tip for steel…the cutting tip has a separate orifice for an oxygen jet which burns thru steel, but It’s not activated unless a lever is pressed…
That generates more heat than is needed but does melting very well… but I have to do it outdoors only…melting and pouring even 2 ounces of silver indoors is dangerous!!!.. any metal that’s accidently spilled on the floor will catch it on fire!!!
…You will need to check the technical specs on a torch that puts out a very large flame with more heat than is sufficient to melt smaller quantities of silver. One that can generate a very large hot flame may not be suited to doing soldering or working on small pieces as it will overheat your work very quickly…high gas output torches will have different regulators from a small one, and higher output knobs… a hardware store bernzomatic propane/air torch with a piezoelectric automatic start using a high output tip for thawing frozen pipes would do the job but not enough to superheat the molten metal…
royjohn is absolutely right when it comes to checking out the technical specs… you can’t extrapolate directly from 2 ounces to 5 however… due to thermodynamic inefficiencies, you will likely need even more BTU’s…
Doing platinum melting is going to be beyond the reach of oxy/propane… it melts at nearly 3,200 degrees F… higher than iron or mild steel and twice as hot as molten silver…that is bright white hot.
That kind of temperature can only be easily achieved using an oxy/acetylene high output industrial grade steel working torch…(I have one that I use for melting) or a high temperature electric furnace that uses an electric arc heater or tungsten filaments… nichrome melts at at 1400 C or about 2800 F… that kind of equipment is industrial and I don’t know whether is been downsized for jewelry applications. Not practical either from safety nor expense…
FWIW, oxy/propane or oxy/ natural gas will weld platinum at the kind of scale we do in jewelry, such as welding the back of a ring shank when sizing. A propane/oxygen flame tops out at about 2820°C/5100°F, natural gas/oxygen at 2770°C/5000°F, and the melting point of platinum is 1768.3°C/3214.9°F. Of course the addition of iridium or ruthenium to the alloy raises the melting point slightly, but it’s still below the temps achieved by propane or natural gas. There’s enough heat to make small welds with a bench top torch or make relatively small melts with a melting torch.
Also, all the shops in NYC run city natural gas/oxygen, and regularly use that for platinum welding and small melts.
How much metal one can melt at once will not be a function of the temperature of the flame, both propane and nat gas burn hot enough, but of how much heat a particular torch can output.
Elliot Nesterman: Thanks for posting that table. You are so correct in that the flame temperature alone does not do the job on it’s own. The volume of burning gas determines its total heat energy output. Also note that the flame temperature is adiabatic. which means that as the hot gas expands at the nozzle, it will cool down further.
There is a table for flame temperatures at the start of this article. Old fashioned coleman fuel blow torches burning naphtha reaches 4,500 degrees F… those of us old enough to remember the old coleman fuel stoves and lamps know that they burned really hot. The oldest formulations of coleman fuel contained benzene, which was removed because it’s a carcinogen… it was replaced by naphthalene, the replaced by naptha which is the equivalent of unleaded gasoline with an octane rating of 55, which is too low for a car engine… these stoves and lamps ran just as well as on unleaded gasoline. The naphthalene and benzene fuels burned as hot as acetylene, because the ratio of carbon to hydrogen in these fuels were 1:1, the same as acetylene… coleman stoves and lamps have all dropped liquid fuel in favor of propane… the latter is much cleaner and easier to use, doesn’t require pumping to maintain pressure and doesn’t require a preheating generator to vaporize the fuel.
The rest of the article is thermodynamics… it shows the theoretic temperature of the flame dropping as the burning gas expanded at constant pressure, ie., atmospheric pressure. That doesn’t make any difference for practical use. The temperatures given in tables for adiabatic burning temperature are at the nozzle, before the burning gas expands…
I’m adding this in just for interest… it doesn’t address the practical needs for soldering and melting but does give you a better idea and a better range of temperatures of combustion for a wider variety of fuels,
If I use the ideal gas law, with pressure in atmospheres, volume in liters, and at a room temperature of 72 degrees (295 K), AND ASSUMING that a small bottle holding 40 grams of oxygen which is 1.25 moles of 02, I get about 225 psi…again ASSUMING that the bottle holds 1.5 liters…which I think is about the size of the small disposable bottle. 1 cubic foot of 02 isn’t much at all. 225 psi isn’t much at all either for compressed gases. It’s my understanding that propane at room temperature is liquid at a pressure of about 150 psi… given your engineering background, did I get the calculation correct or am I wrong?
another way of looking at the 40 grams of oxygen, is that the volume of oxygen in the bottle is 1 cubic feet or 28.6 liters, if decompressed to atmospheric pressure. A mole of an ideal gas would take up 22.6 liters at standard temperature and pressure. the same number of moles, 1.26 would make up a cubic foot of gas… the ideal gas law, which was used for the first calculation would come up with the same pressure, about 225 psi for a 1.5 liter bottle… ? QED…
The table gives you the heat output in BTU’s per hour for different tip sizes… the largest one puts out enough heat to melt 3 ounces of silver. , create less unwanted heat, as they heat only the metal DIRECTLY, some are capable of melting platinum…The downside is that many require 240V and are expensive. The cheaper ones do not have closed melting chambers and lose heat as well as being open and exposed to the air. They also will not melt platinum.
Trying to get useful technical specs off the internet is nearly impossible… Amazon lists stuff for sale without providing specs…The only real way to get the full technical specs is to contact the manufacturer…Ro Grande can give you specs on the products they sell.
Sorry, for some reason, the table was cut out… the #7 tip for a little smith torch produced a little over 9,000 BTUs per hour, enough to melt 3 ounces of silver. That’s the largest tip available in standard, but there also is a multi orifice blow torch head for melting. It’s oxy propane.

MUST HAVE – MultiJet casting nozzle.
Undoubtedly the best addition to the Smiths Little Torch is the MultiJet casting nozzle. Two are available, one for Propane and one for Acetylene. The nozzle produces a large wide flame at the end of an extra-long nozzle. With a soft bushy flame, this is ideal for annealing metal, such as bangles, bars, and coils of wire. With more Oxygen, the MultiJet nozzle can melt down several ounces of silver in a matter of minutes. Ideal for small castings, and recycling metal with a rolling mill. The extra length also keeps you and the torch away from the heat. So, don’t think the Smiths Little Torch is just for extra fine work. With the right nozzle it can melt almost anything.
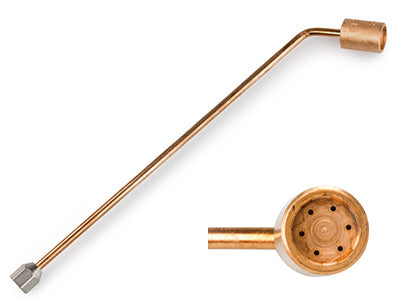
The Smiths Little Torch multi-jet casting nozzle.
Note there are two versions, Propane and Acetylene.
This is what I have and what I’m currently using. It does ok, but the specs say it maxes out at 3 ounces. I’ve heard a lot of people say that it won’t even do that much. I haven’t tried to do more than 2 ounces with it. It takes forever, but it will work.
turn both the gas and oxygen up as much as you can without having it hissing. A leaner flame, more oxygen burns hotter. If it’s taking that long to melt you might just try a plain old Bernzomatic propane air blow torch with a large capacity burner head… it takes long to melt also and won’t give you much superheat…but it’s cheaper than using up compressed oxygen… I’ve never bothered using oxy-propane to melt, since I have the oxyacetylene torch. It melts in seconds… instantly almost… I have used the same torch with propane, but have to dial back on the regulators and gas flow to prevent overheating. …even though the torch is not rated for propane. using propane does burn up a lot more oxygen relative to acetylene, But compressed oxygen is fairly cheap. Acetylene isn’t. When using propane, I have it hooked up to a 20lb gas barbecue grill bottle… you can’t get cheaper propane than that…why do you need to melt so much silver? are you doing large castings or casting ingots for rolling in a rolling mill for sheet and wire? 2 ounces should be more than enough to cast an ingot for rolling.
Hi Steve, I have been away on vacation so have not had a chance to answer you. I am not sure why you need to know the pressure, but since you asked… If I had to do a quick guess at the pressure I would use the ideal gas law and simplify it to P2 = P1xV1/V2. P1 is 14.7 PSIa, V1 is 1 ft3 which is the volume of 1.25 moles of gas at STP. You had suggested that the volume of the cylinder is about 1.5 liters or say 0.053 ft3. Then solving for P2, P2= 277 psia or about 262 psig. The solution is highly dependent on the internal volume of the cylinder. Also, the ideal gas law only works at low pressures. As the pressure increases, the gas becomes non-ideal because the gas molecules are getting closer together and interacting with each other. A more sophisticated calculation is required to take into account the non-ideality of the gas and get a more accurate assessment of the pressure. I have not had to do this with a hand calculation in over 40 years. In industry we have very sophisticated tools available to us to calculate gas properties in non-ideal conditions and unfortunately I do not have access anymore. So in summary, this is just a rough estimate of the pressure.
thanks for the insight. I agree that the ideal gas law works only for small gas atoms of molecules at low pressures. Higher pressures squeeze them closer together… the van der wal correction can account for some non ideal behavior, but if I remember correctly, it’s still at lower pressure. The reason that I asked about the pressure is that it is related to how much gas is compressed in the cylinder. Disposable and propane cylinders aren’t built to withstand the much higher pressure that commercial gas bottles hold. So using those disposable O2 cylinders is highly cost ineffective and should be avoided… I have a small, 150 cubic foot commercial 02 bottle… it lasts long enough for a calculated 14 hours of cumulative use with propane as the fuel…starting at a pressure of 2000 to 2200 psig…oxygen concentrators on the other hand, can generate an indefinite amount of 02 albeit at low flow rates… as I understand it, a synthetic zeolite filter adsorbs nitrogen, while another one is being purged of N2… they don’t last forever because eventually they do become saturated and can’t be purged… how the zeolite filter is purged of nitrogen however is something that I don’t understand… the process apparently uses atmospheric air, which for an approximation is about 80% N2…without some process such as heating to drive off the adsorbed N2, there won’t be a thermodynamically favored path to freeing the adsorbed N2… does anyone out there know how this works?
I don’t have a degree in chemistry nor physics… just a couple of semester of each, 50 years ago… I’m just an interested lay person who happens to like science, particularly chemistry…
All right I’m going to do this. I think I also figured out part of the problem: the crucibles I have are too big and too thick. I think with smaller, thinner crucibles I should be able to melt the metal faster and to a more liquid state for casting. I haven’t tested this theory yet, but it makes sense. Large things suck up more heat. I bought some smaller crucibles, different kinds, I’m going to season them and then try to melt in them and see how it goes. At the moment, I’m doing a lot of sheet and wire, yes. The problem I’m running into with the wire is the steel reversible ingot mold I got doesn’t make very big wire ingots. So I got an actual wire ingot mold that’s cast iron. I want some very thick wire to make larger sized comfort fit ring shanks, and the little steel ingot mold just can’t do it. Unfortunately the stupid wire mold I bought came covered in a type of rubber or silicone paint (why?!?!) and has a lot of pits in it. I can’t burn the paint off as others have suggested, that creates fumes. So I’m going to have to sand it off and also smooth out the pits. Which is probably going to take forever, but c’est la vie.
burn off the junk outdoors…and stay upwind of the fumes… have to get it really hot to do that and heating an ingot mold takes prolonged heating… in your case, heat it until it stops smoking or fuming…
I use both a propane blow torch and oxyacetylene full sized steel working torch to heat it up for a pour… you can put oil or vaseline in the mold, but it has to be smoking hot to pour… good luck with manually cleaning the gunk… burning it up first might make it easier…even throw the thing on a bed of charcoal in a hibachi or other charcoal grill… that will make it very hot… and not waste a ton of gas.
I use a thick melting dish with fireplace tongs that have a circular part that I can use to grab the dish securely.,.I always use oxyacetylene to melt… it gets the metal very hot, reddish yellow to yellow which is about 2000 degrees F…superheat makes the metal pour fully…it very fluid… I’ve had to remelt the pour on a couple of occasions because the mold wasn’t hot enough and the silver solidified only part way in. My ingot mold does make rods thick enough for a thin shank…
I’ve found that dishes work better than thin deep pouring crucibles… if the crucible is too deep, there will be a lot of flame blowback that prevents the heat from directly melting the metal…
my problem is that the borax flux gets very thick and pasty which makes it difficult to clean… do you have any ideas about cleaning a crucible that is gunked up with borax?
Dana, Hans Meevis designed and sells a superior ingot mold, demonstrated here by Andrew Berry:
Neil A
So I’m gonna start at the end of your post here. I watched a thing from Andrew Berry on how to clean crucibles. Basically you just melt it out. Same process as if you were melting metal except you’re after the borax. He said the easiest way to do this is to melt metal, pour metal, then clean the crucible out right then and there because it’s already really hot. I watched that before I started goldsmithing so I just do that every time now, I melt all the excess borax out of the crucible right after I pour and it keeps them pretty cleaned out. My problem has been borax mixing in with and sticking to the ingot. Like every single time. It’ll be marbled in hard borax to the point that it affected the way the ingot solidified so it’s not perfectly solid and level. Putting it in the pickle doesn’t help all that much because it’s so thick. Watching videos, I’ve never seen anyone else have this problem. I have to take a large file and whack the crap out of the ingot to break the excess borax off, even file some of it off, then I can start rolling the mess down. I can’t figure out why it’s doing this. I’ve watched videos of people adding a TON of borax to their metal and their pour doesn’t look anything like mine, so I’m not sure if it’s because I’m using too much borax or what, but it’s been really aggravating. It’s the reason I haven’t done any casting except for ingots so far because if I spend a bunch of time carving something and then cast it and it comes out marbled with borax, I’m gonna be really upset. I have to figure out how to fix this problem before moving on.
I spent last weekend burning off paint and sanding down stuff. I’ve given up on that ingot mold for now, it’s terrible. Bumpy, pits, uneven, and I think it’s way too big for what I’m doing because it takes forever to heat it up. I bought a smaller one that was machined smooth and even, and it gets the job done. But I’m seriously starting to just prefer graphite molds. You don’t have to oil them. They’re always perfectly smooth, they come in different shapes, and the metal dumps out right away. Easy.
I’m really paranoid about thermal flashback so I will sit there and spend more time heating the mold than I do the metal. It’s impossible to tell if the mold is hot enough. My heart starts racing when it’s time to pour lol. Like if anything cracks or pops I’m so on edge that I jump so I hold the crucible tongs as little as possible because it would be just my luck something superficial pops and I jump and get covered in molten metal lol. Scares the crap out of me. I think it’s getting better, though, I’m getting more comfortable with it.
I love Andrew Berry! He talks fast and packs in a lot of info, really in-depth. I haven’t watched this video, but I’ve seen it in the list before. Thank you!!!
